Abstract
Background: Haploidentical Hematopoietic Stem Cell Transplantation (Haplo-HCT) is an established therapy using alternative donors for patients with Acute Myeloid Leukaemia (AML) and Myelodysplastic Syndromes (MDS). There is a limited body of evidence for older patients undergoing Haplo-HCT in AML and MDS. Studies describing Haplo-HCT in older patients have used a high proportion of bone marrow (BM) derived grafts and a variety of conditioning regimens. In this setting, Haplo-HCT demonstrates acceptable Non-Relapse Mortality (NRM) and chronic Graft Versus Host Disease (cGVHD) rates of less than 10% (Ciurea. Biology of Blood and Marrow Transplantation. 2018 Jun 1;24(6):1232-6.). In Australia and New Zealand, Haplo-HCT is predominantly performed using peripheral blood (PB). We performed a retrospective national registry study to examine the outcomes of Haplo-HCT using PB in older patients.
Methods: Data was collected through the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR) for patients aged 65 or older receiving a PB Haplo-HCT for AML/MDS between January 2010 and July 2020. Cumulative incidence functions were used for engraftment, CMV reactivation, acute GVHD, chronic GVHD, relapse, and NRM. The competing risk for engraftment, CMV reactivation and GVHD was death. Relapse and NRM were competing risks for each other. Overall survival (OS), relapse-free survival (RFS), and composite GVHD and RFS (GRFS) were calculated using Kaplan-Meier analyses. The impact of pre-transplant factors on these endpoints was analyzed using Cox regression.
Results: A total of 44 patients were included in the analysis. The median follow-up time was 734 days. The median age was 68 (range 65-74) with a median Karnofsky Performance Status of 90. Thirty patients (68.2%) had AML while 14 (31.8%) had MDS. The median donor age was 40. The most common conditioning regimen was non-myeloablative fludarabine, cyclophosphamide and TBI (73.8%), the remainder of the patients received either melphalan or busulfan based regimens, the majority were reduced intensity with only 2 patients undergoing myeloablative conditioning. All patients received post-transplant cyclophosphamide and mycophenolate mofetil with the majority also receiving tacrolimus (90.5%) and the remainder receiving cyclosporin (9.5%). No patients received anti-thymocyte globulin.
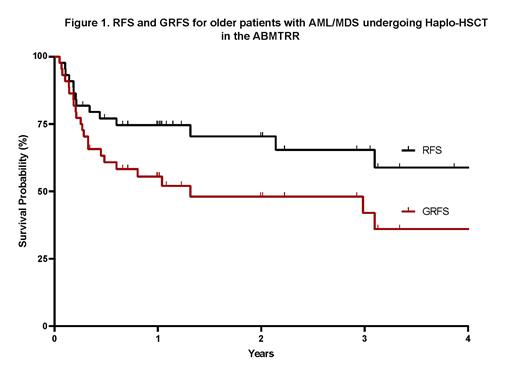
Neutrophil engraftment was achieved in 97.6% of patients, at a median of 18 days while platelet engraftment was achieved in 92.7% of patients at a median of 28 days. The cumulative incidences of CMV reactivation and CMV disease were 52.5% and 5.1% at 1 year The incidence of Grade 2-4 aGVHD was 18.2%. The incidence of cGVHD at 2 years was 40.7%, with extensive cGVHD occurring in 17.7% of patients. The incidences of relapse and NRM at 2 years were 8.8%. and 20.7% respectively. The leading causes of death were infection (64.7%) followed by relapse (14.2%). The 2-year OS was 74%. RFS and GRFS at 2 years was 70% and 48% (Figure 1). Recipient age, donor age or disease type (AML vs MDS) had no significant impact on OS or GRFS.
Conclusion: These results confirm the safety and effectiveness of Haplo-HCT for AML/MDS in older patients. The rates of cGVHD were higher than expected given the lower rates reported in other studies using PTCy GVHD prophylaxis. Haplo-HCT using a PB graft demonstrates good long-term disease control with reasonable rates of NRM and cGVHD for older patients with AML/MDS.
Bajel: Abbvie, Amgen, Novartis, Pfizer: Honoraria; Amgen: Speakers Bureau. Perera: Abbvie: Speakers Bureau; BMS: Speakers Bureau. Greenwood: Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hamad: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal